Introduction: The Life-Saving Drug That Can Quietly Erase Your Immune Memory
Modern medicine has created therapies so powerful they can stop autoimmune destruction in its tracks, calm aggressive inflammation, and even save lives in cancer.
But sometimes, the very treatment that protects one part of the body can silently weaken another.
In this episode of Wellness Focused, Dr. Bismah Irfan, MD, uncovers a critical and often overlooked consequence of anti-CD20 therapies such as:
- Rituximab (Rituxan)
- Ocrelizumab (Ocrevus)
- Ofatumumab (Kesimpta)
These medications are widely used in autoimmune diseases, kidney disease, neurologic conditions, and certain cancers. They can be life-changing-and for many patients, they are absolutely necessary.
But here’s the issue most patients are never warned about:
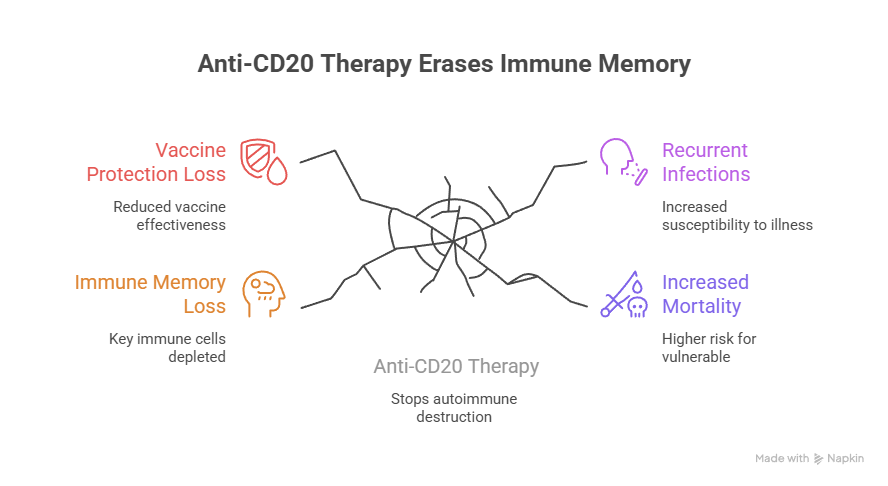
Anti-CD20 therapy can erase immune memory-and in many people, it may not fully return.
This isn’t just a theoretical risk. Emerging long-term research shows persistent depletion of key immune cells years after treatment ends, loss of vaccine protection, recurrent infections, and increased mortality in vulnerable patients.
If you’ve ever taken anti-CD20 therapy-or are considering it-this blog will help you understand what’s happening inside your immune system and what you can do to protect yourself.
What Anti-CD20 Therapy Does (In Simple Terms)
Anti-CD20 therapies work by targeting a marker called CD20, found on the surface of B cells.
B cells are immune cells responsible for:
- Producing antibodies
- Coordinating immune responses
- Creating long-term immune memory
- Responding to vaccines
When anti-CD20 therapy is given, it depletes B cells, often dramatically.
This can be beneficial because many autoimmune diseases are driven by dysfunctional B cells that:
- Produce harmful antibodies
- Activate inflammatory cascades
- Trigger immune attacks on organs
In kidney disease, these therapies may be used for conditions like:
- Lupus nephritis
- ANCA vasculitis
- Membranous nephropathy
- Other immune-mediated glomerular diseases
So yes-anti-CD20 therapy can be life-saving.
But immune suppression is never “free.”
The Hidden Problem: Not All B Cells Are the Same
Many doctors assume that once B cells “come back,” the immune system recovers.
But that assumption is often incorrect because there are different types of B cells, and they don’t all regenerate equally.
Key B Cell Types
- Naïve B cells: “Young” B cells that haven’t learned anything yet
- Memory B cells: Long-lived cells that remember past infections and vaccines
- Switched memory B cells: Highly specialized memory cells critical for durable immunity
- Plasma cells: Antibody factories that can survive for years
Anti-CD20 therapy wipes out CD20-positive B cells-but recovery is uneven.
Why Immune Recovery Feels Incomplete
One of the most important findings from long-term research is this:
Naïve B cells often return, but switched memory B cells may not.
This creates a dangerous illusion of recovery.
A patient’s B cell count may look “normal,” but their immune system may still be missing the most important cells for real protection-the ones that remember.
That means the immune system can look rebuilt on paper, while remaining functionally compromised in real life.
Immune Memory Loss: What That Actually Means
Immune memory is the body’s ability to recognize threats it has seen before and respond quickly.
Without immune memory:
- Infections hit harder
- Illness lasts longer
- Recovery is slower
- Complications increase
- Vaccines become less effective
This is why people can feel like their immune system “never bounces back” after treatment-even years later.
The Vaccine Problem: Why Protection May Be Permanently Reduced
One of the most concerning consequences of anti-CD20 therapy is vaccine failure.
Vaccines work by training B cells to:
- Recognize pathogens
- Create antibodies
- Form memory cells for long-term protection
If B cells are depleted or memory B cells fail to regenerate, vaccine immunity can fade.
Dr. Irfan highlights emerging research showing:
- Loss of antibody protection to vaccines patients previously responded to
- Poor response to new vaccines during and after therapy
- Revaccination often fails to restore adequate antibody levels
This means patients may believe they are protected-when they are not.
And the risk isn’t limited to COVID vaccines. It may include reduced protection to:
- Influenza
- Pneumococcus
- Hepatitis
- Varicella (chickenpox/shingles)
- Tetanus and others
The Real-World Consequences: Infections, Hospitalizations, and Mortality
Immune memory loss isn’t just an inconvenience-it can be dangerous.
Long-term studies show that some patients experience:
- Recurrent sinus infections
- Bronchitis and pneumonia
- Persistent viral illnesses
- Opportunistic infections
- Increased need for antibiotics
- Higher hospitalization rates
In severe cases, chronic immune suppression is associated with increased mortality-especially in older adults and those with overlapping conditions.
For kidney patients, this risk is amplified because CKD itself causes immune dysfunction.
So when you combine:
- CKD-related immune weakness
- Anti-CD20-related B cell depletion
- Other immunosuppressants
…the result can be a profoundly vulnerable immune state.
Why Do Memory B Cells Fail to Recover?
Dr. Irfan explains several reasons why switched memory B cells may not regenerate properly.
1. Bone Marrow and Immune Stem Cell Stress
B cells originate in the bone marrow. If the bone marrow environment is damaged-by inflammation, illness, medications, or age-B cell regeneration may be incomplete.
2. Chronic Inflammation
Inflammation disrupts immune signaling and can block healthy immune rebuilding. Many patients receiving anti-CD20 therapy already have chronic inflammation from autoimmune disease.
3. Repeated Dosing
Many patients receive multiple rounds or long-term maintenance dosing. Each round may prevent full immune rebuilding from ever occurring.
4. Overlapping Immunosuppression
Patients often receive anti-CD20 therapy along with:
- Steroids
- Chemotherapy
- Mycophenolate
- Cyclophosphamide
- Calcineurin inhibitors
This layered suppression can severely impair immune recovery.
5. Age and Immune Aging
Immune regeneration declines with age. Older patients are less likely to fully rebuild memory B cell populations.
The Biggest Blind Spot: Most Patients Aren’t Monitored Properly
Here’s one of the most alarming realities Dr. Irfan highlights:
Most patients on anti-CD20 therapy never have their immunoglobulins or B cell subsets monitored.
This means patients may become immunodeficient silently, without knowing it-until they develop repeated infections or serious complications.
Many clinicians track:
- CBC
- Kidney labs
- Liver enzymes
But do not routinely track:
- Immunoglobulin levels (IgG, IgA, IgM)
- B cell subsets (including memory B cells)
- Vaccine antibody titers
So patients are left unprotected, without warning.
What Immune Monitoring Should Include
Dr. Irfan emphasizes that immune recovery should never be assumed-it should be measured.
Core Monitoring Labs
- IgG, IgA, IgM
- B-cell panel / lymphocyte subsets (including CD19/CD20 counts)
- Memory B cell subsets (especially switched memory B cells)
Additional Useful Testing
- Vaccine titers (to assess protection)
- Infection history review
- Vitamin D and zinc status (immune cofactors)
Monitoring doesn’t mean stopping life-saving therapy.
It means preventing avoidable complications.
How Patients Can Advocate for Themselves
If you’re receiving anti-CD20 therapy, Dr. Irfan encourages you to ask your doctor:
- Have we checked my IgG, IgA, and IgM levels?
- Are my B cells recovering-or only naïve B cells?
- Do I still have vaccine immunity?
- Should I see immunology for monitoring?
- What is the plan if my immunoglobulins stay low?
This is not “being difficult.”
This is being informed.
What Can Be Done If Immune Recovery Is Poor?
Every patient is different, but options may include:
- Adjusting treatment frequency when clinically safe
- More careful infection prevention planning
- Considering immune support strategies
- Referral to immunology for advanced management
- Monitoring for the need for antibody replacement therapy in select cases
The key is early detection-before complications occur.
The Kidney Patient Factor: Why This Matters Even More in Nephrology
Kidney disease patients are already at higher risk of:
- Infections
- Inflammation
- Immune dysfunction
- Poor vaccine response
So when anti-CD20 therapy is added, the immune consequences can be magnified.
That’s why kidney care must include immune strategy-not just creatinine and proteinuria tracking.
Three Key Takeaways
1. Anti-CD20 Therapy Can Cause Long-Term Immune Memory Loss
Studies show memory B cells may remain depleted for years after treatment, impairing the immune system’s ability to respond to infections and vaccines.
2. Vaccine Protection May Be Permanently Reduced
Many patients lose immunity to prior vaccines, and revaccination often fails to restore protective antibody levels.
3. Immune Recovery Should Never Be Assumed
Without routine immunoglobulin testing and B-cell monitoring, patients may face recurrent infections and serious complications without warning.
Conclusion: Your Immune System Deserves the Same Attention as Your Disease
Anti-CD20 therapies have transformed modern medicine. They save lives, protect organs, and offer remission where there was once relentless progression.
But they come with a cost that is often ignored:
Immune memory is not guaranteed to return.
If your immune system feels weaker long after treatment, it may not be in your head-and it may not show up on routine labs.
The solution is not fear.
The solution is monitoring, awareness, and advocacy.
Because when you understand what anti-CD20 therapy can do long-term, you can take proactive steps to protect yourself-while still receiving the treatment you need.
Your immune recovery should never be assumed. It should be measured.
